Who we are
The Childhood Acute Illness and Nutrition Network (CHAIN) is a global research network focused on optimizing the management and care of highly vulnerable children in resource-limited settings to improve survival, growth and development.
The Childhood Acute Illness and Nutrition Network (CHAIN) is a global research network focused on optimizing the management and care of highly vulnerable children in resource-limited settings to improve survival, growth and development.
What is our goal?
The CHAIN Network aims to identify the biological mechanisms and the socio-economic factors that determine a child’s risk of mortality in the six months following presentation to medical care with an acute illness. Ultimately, The CHAIN Network aims to improve care for acutely ill children living in countries with limited resources and prevent both in-hospital and post-discharge mortality. To do this, the progress of acutely ill children across a range of nutritional status will be tracked throughout their hospital stay and for 6 months after returning to their home and communities. This research will guide the choice of strategies to be taken forth into clinical trials to reduce mortality in this highly vulnerable population.
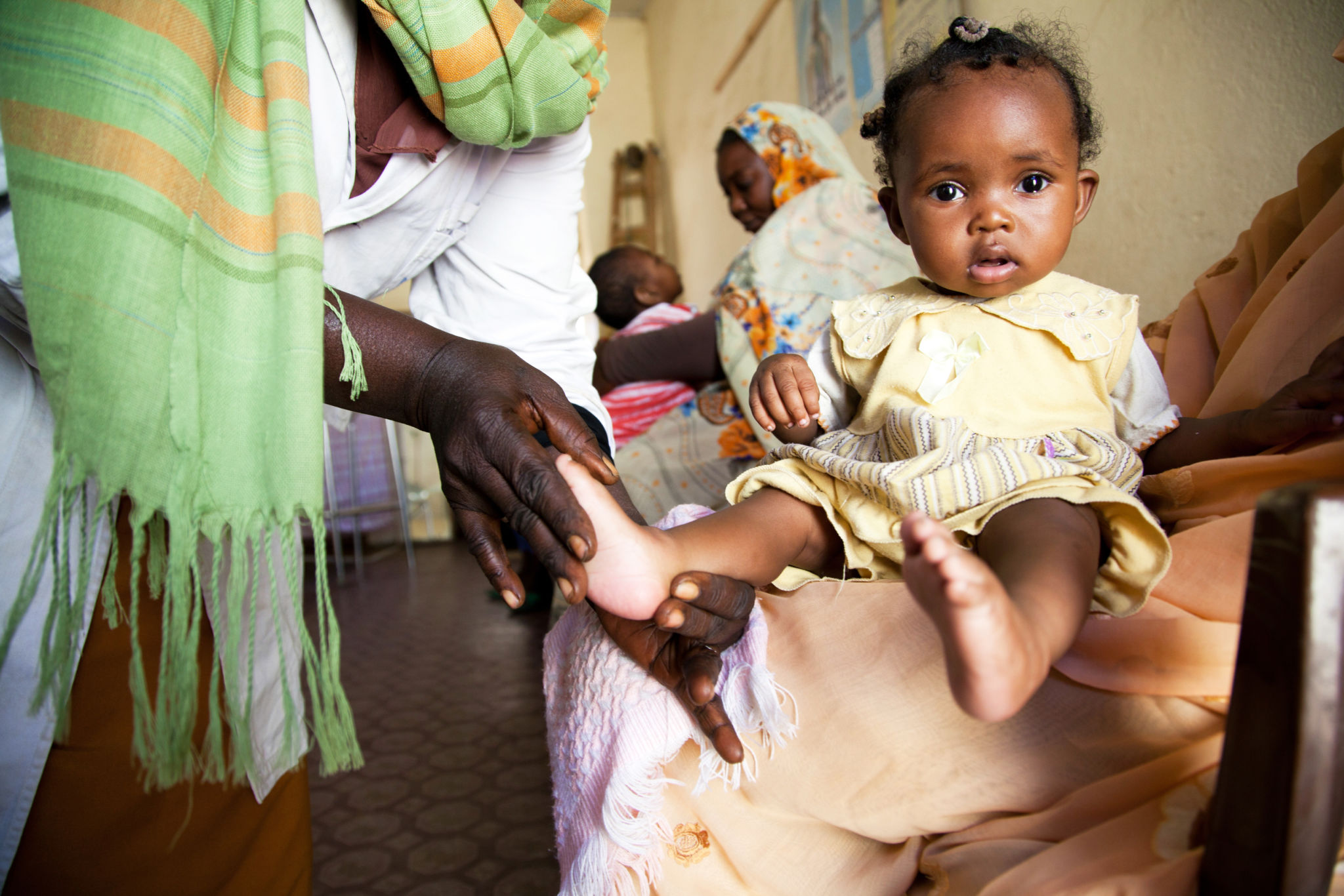
Despite an overall reduction of child mortality in low and middle income countries, acutely-ill undernourished children continue to have a greatly elevated risk of death, both during hospitalization and following discharge. However, we currently lack robust evidence to guide optimal management. The ultimate goal of the CHAIN Network is to identify and prioritize actionable intervention time points and targets to reduce mortality amongst acutely ill undernourished children.
We aim to understand the complex factors that increase mortality, hospital readmission and poor recovery in young acutely ill children. These include biological factors (e.g. infections, immune responses and metabolism), nutritional factors (e.g. breast-feeding, diet and food security), health systems (e.g. accessibility to care and clinical management) and sociological factors (e.g. economic constraints, income loss, time spent with/away from the child, mental health and care seeking behavior).
Approximately 5 000 young children between the ages of 2 and 23 months will be recruited. Each site will follow ~500 children admitted to hospital for an acute illness who have varying degrees of malnutrition i.e., being severely to moderately to not malnourished. Additionally, each site will study 125 non-acutely ill children living within the community of the hospital catchment area.
This study aims to improve the care, survival and life quality of ill children living in countries with low resources. Findings from this research will directly inform guidelines and policies related to the management and care of highly vulnerable children and will help prioritize interventions that warrant further testing in clinical trials.

The initial core activity of the CHAIN Network is to establish a large multi-site cohort study of hospitalized children under 2 years old, and to follow these children for 6 months after they are discharged. This cohort study aims to better understand the biological and social reasons that cause malnourished acutely ill children to die at such high rates during and after hospitalization. The findings of this cohort will be strengthen by a range of specialist sub-studies. The CHAIN Network will use this information to prioritize and guide the design of future clinical trials that will help reduce mortality in these vulnerable children.
Four thousand eight hundred children will be recruited at admission to hospital across nine sites. We will follow these children from admission and then daily throughout their hospital stay, and then will be followed up at 45, 90 and 180 days after their discharge. An additional 100 children living in the community will be recruited to participate at each site. These community controls will help establish reference norms for various laboratory tests which currently lack accepted normal threshold values.
Investigators from the CHAIN Network are also involved in a number of on-going clinical trials that will contribute to the Network’s overarching goals. The results obtained from these trials will be used to prioritize future clinical trials. Two particularly relevant ongoing clinical trials are:

In order to expedite the scientific process, The CHAIN Network aims to capitalize on pre-existing biological samples that have been collected in the context of two recently completed clinical trials. The first trial was designed to assessed the prophylaxis use of co-trimoxazole antibiotic given post-discharge as method of reducing mortality among children with severe acute malnutrition after they are discharged from hospital. Results from this trial were recently published in the Lancet Global Health. The second was the F-75 clinical trial conducted to test a novel macro nutrient formulation of F-75, the feed given to children with severe malnutrition during acute illness.
Analyzing these pre-existing samples will help The CHAIN Network to rapidly test key hypotheses which will guide the prioritization of interventions to reduce inpatient and post-discharge mortality.